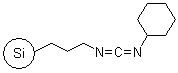|
|
 |
Silica-Bound Reagents
“Although we now have some excellent methods to effect the construction of target molecules with an ever increasing level of complexity, there is a need to find new, strategically important processes which are environmentally cleaner, more efficient and which lead to greater structural variation in a shorter period of time”
S. V. Ley et al., J. Chem. Soc., Perkin Trans. 1, 2000, 3815
What is special about solid-supported reagents?
Solid-supported reagents provide an attractive and practical means of expediting organic synthesis. The advantages of using bound (i.e. insoluble) reagents instead of their solution phase counterparts in organic synthesis are as follows:
- Toxic or difficult to remove reagents and by-products are immobilised and can be separated from the product by a simple filtration
- Purification protocols including chromatography and liquid-liquid extractions, which are time consuming and difficult to scale up, are not required
- A large excess of reagent can be used, thus driving the reaction to completion
- One-pot multiple step reactions are easier to carry out
- They are suitable for use in flow-through applications and automated synthesis
The advantages of functionalised silica over polymer-supported equivalents
Silica-bound reagents are a class of modified silica gels that possess reactive moieties and the capacity to conduct chemical transformations. As illustrated in Figure 1, these materials are produced via surface functionalisation of the polar silanol (Si-OH) groups of regular silica.
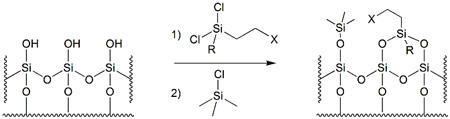
Figure 1 – Surface functionalisation of regular silica gel, followed by end-capping
Silica-supported reagents and scavengers have a number of distinct advantages over their polymeric counterparts:
- Fast Kinetics: since the silica particles are surface functionalised, the rate of reaction is not controlled by diffusion in and out of the polymer. Most SiliaBond® scavengers exhibit reaction times of less than 60 minutes.
- Solvent Independent: Silica neither shrinks nor swells in any solvent and, because it is end-capped, it does not partially dissolve in any solvent either.
- Easy to use: Silica does not carry a static charge; it is free flowing and thus easy to weigh out and handle. Its high density makes it suitable for small volume work, it does not require extensive washing for high recoveries and it won't stick to glassware!
- Thermally Stable: Most SiliaBond® reagents and scavengers can withstand temperatures of over 200
°C and are suitable for use in microwave synthesisers.
- Flexible Formats: Since it does not swell, silica can be packed into flow through systems including HPLC columns, Flash & SPE cartridges and 96 well plates.
SiliaBond® reagents for organic synthesis
SiliCycle Inc. is a pioneer in the development of functionalised silica gels, gathered under the name SiliaBond®, for use in synthesis and purification. The backbone of all SiliaBond® products is stationary phase silica gel having particle size 40 – 63 µm and pore size 60 Å.
The following table provides information on the SiliaBond® reagents. All reagents and scavengers are available in Flash, SPE and 96 well plate format. Please contact us with your enquiry or to request a quote.
| Manufacturer |
SiliCycle® Inc. |
| Availability |
from 10 g to 25 kg |
| Particle Size |
40 - 63 µm (mesh 230 - 400) |
| Pore Size |
60 Å |
| Specific surface area |
500 m2/g |
Product Code |
Name |
Structure |
R74530B |
SiliaBond® Aluminium Chloride (nec) |
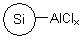
|
- Catalyst for Friedel-Crafts acylations and alkylations1-3, and for the synthesis of ethers (SiliCycle 2008 catalogue, page 71)
- In comparison to free AlCl3, the silica-bound equivalent offers milder acidity, superior shelf-life and the ability to conduct non-aqueous work-ups4,5
- Loading: 1.6 mmol/g; compatible with all organic solvents
|
R52030B
R52130B |
SiliaBond® Amine
SiliaBond® Amine (nec) |

|
- Solid-supported primary amine base; can be used as a catalyst for Knoevenagel condensations6 and Claisen rearrangements7
- Useful as: a stationary phase for the separation of peptides, metabolites, steroids, cholesterols, triglycerides and sugars; a weak anion exchanger for the purification of strong acids; and as a supported scavenger of electrophiles & metal complexes
- Loading: 1.6 mmol/g; compatible with all solvents (aqueous and organic)
|
R70530B |
SiliaBond® Carbodiimide |
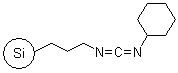
|
- Used in amide and Weinreb amide syntheses (SiliCycle 2008 catalogue, pages 64 and 84); also applicable in acylsulfonamide synthesis (SiliCycle 2008 catalogue, page 81) and in esterification reactions including synthesis of activated esters
- Loading: 1.0 mmol/g; not compatible with protic solvents
|
R66030B |
SiliaBond® Carbonate |
|
- Solid-supported tetraalkyl ammonium base, used to free base amines in their ammonium salt form8 (SiliCycle 2008 catalogue, page 67)
- Also useful as a scavenger of acids and acidic phenols including HOBt,9 which is commonly used in amide coupling reactions (SiliCycle 2008 catalogue, page 77)
- Loading: 0.7 mmol/g; compatible with all solvents (aqueous and organic)
|
R66730B |
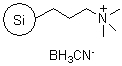
SiliaBond® Cyanoborohydride |
|
- Silica-bound equivalent of sodium cyanoborohydride, used in: reductive aminations (SiliCycle 2008 catalogue, page 73 ); the reduction of imines and aldehydes; and the reduction of α,β-unsaturated carbonyl compounds
- Loading: 1.0 mmol/g; compatible with all solvents (aqueous and organic)
|
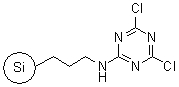
R52230B |
SiliaBond®
Dichlorotriazine
|
|
- Silica-bound equivalent of 2,4,6-trichloro-1,3,5-triazine
- Used in the synthesis of amides, Weinreb amides (SiliCycle 2008 catalogue, pages 85 and 86) and acylsulfonamides (SiliCycle 2008 catalogue, page 81)
- Loading: 0.7 mmol/g; compatible only with anhydrous aprotic solvents
|
R76530B |
|
|
- Can be used as a solid-supported tertiary amine base for Knoevenagel and Michael reactions (the by-products are immobilised and easily removed by filtration)
- Also useful as a stationary phase for ion-exchange chromatography and as a weak anion exchanger for solid phase extraction (SPE) and HPLC
- Loading: 1.2 mmol/g; compatible with all solvents (aqueous and organic)
|
R45030B |
|
|
- Can be used as a solid-supported tertiary amine base for the catalysis of Michael10 and Knoevenagel11 reactions ; also applicable in alkane oxidations12
- Unlike polystyrene-based equivalents, the silica-bound amine is not susceptible to cleavage by electrophiles
- Loading: 1.4 mmol/g; compatible with all solvents (aqueous and organic)
|
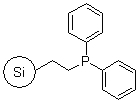
R39030B |
SiliaBond® Diphenylphosphine |
|
- Can be used as a linker for the immobilisation of catalysts including Pd(PPh3)4 for metal catalysed coupling reactions (SiliCycle 2008 catalogue, page 83)
- Loading: 0.9 mmol/g; requires degassed solvents
|
R75530B |
SiliaBond® DMAP |
|
- Silica-bound equivalent of 4-dimethylaminopyridine, used as a catalyst in numerous transformations including acylations, amidations and acetylations
- Unlike its free counterpart, SiliaBond® DMAP can be removed from the crude reaction mixture by filtration
- Loading: 0.8 mmol/g; compatible with all organic solvents
|
R60030B |
SiliaBond® Piperazine |
|
- Deprotecting and scavenging agent for Fmoc13 and Bsmoc14 protecting groups; also used as a solid-supported catalyst for Knoevenagel reactions
- According to published research, silica-supported piperazine works more effectively than the polystyrene-based equivalent13
- Loading: 0.8 mmol/g; compatible with all solvents (aqueous and organic)
|
R23030B |
SiliaBond®
Potassium Permanganate
(nec) |
|
- Oxidises methyl- and alcohol-functionalised molecules to the corresponding carboxylic acids, while the manganese by-product stays adsorbed onto the silica
- Solid-supported KMnO4 increases recoveries, facilitates work-up and expands the scope of the chemistry because it can be carried out in non-aqueous solvents15
- Loading: 20% wt/wt, adsorbed onto silica; not compatible with alcohol-, aldehyde- and ketone-functionalised solvents
|
R51230B |
SiliaBond®
Propylsulfonic Acid
|
|
- Silica-bound sulfonic acid that is used as a general acid catalyst and solid-supported base scavenger
- Can be used to provide an acidic work-up in the absence of aqueous conditions
- Loading: 1.0 mmol/g; compatible with all solvents (aqueous and organic)
|
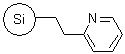
R43030B |
SiliaBond® Pyridine |
|
- Can be used as a solid-supported tertiary amine base, as a scavenger of acidic molecules and as a Cu2+ anchor in the oxidation of hydroquinones16
- Loading: 1.3 mmol/g; compatible with all solvents (aqueous and organic)
|
R24030B |
SiliaBond®
Pyridinium Chlorochromate (nec) |
|
- Versatile solid-supported oxidant17-20; used in the general oxidation of alcohols to carbonyl compounds, selective oxidation of allylic and benzylic alcohols, organometallic oxidations, oxidative transpositions, oxidative cleavages, allylic and benzylic oxidations, and oxidative cyclisation reactions
- The use of immobilised pyridinium chlorochromate provides anhydrous conditions that disfavour side-reactions and increase yields; moreover, it facilitates removal of chromium by-products and is compatible with acid-sensitive protecting groups21,22
- When used in conjunction with ultrasound technology, the kinetics are increased and the amount of oxidant required to complete the reaction is reduced23-25
- Loading: 20% wt/wt, adsorbed onto silica; compatible with anhydrous CH2Cl2
|
R24530B |
SiliaBond®
Pyridinium Dichromate (nec) |
|
- Used as an alternative to SiliaBond® pyridinium chlorochromate in nucleoside and carbohydrate oxidation reactions, particularly for fragile molecules26; also used in conjunction with tert-butylhydroperoxide for a variety of oxidative transformations27
- Solid-supported pyridinium dichromate is a convenient and effective reagent for the oxidation of allylic and benzylic alcohols, as well as saturated primary and secondary alcohols; moreover, it is compatible with acid-sensitive groups such as ketals28
- Loading: 20% wt/wt, adsorbed onto silica; compatible with anhydrous CH2Cl2
|
R68530B |
SiliaBond® TBD |
|
- Versatile solid-supported base, catalyst and reagent29,30
- It is sufficiently basic to deprotonate moderately acidic hydrogens and has been used in Michael reactions, Knoevenagel condensations, Robinson annulations and nucleophilic epoxidations
- As a reagent it can be used in the alkylation of phenols (Williamson ether synthesis) and amines, esterification of carboxylic acids by use of alkyl halides, alkylation of activated methylene-containing compounds, dehalogenation of organic halides and synthesis of aryl triflates
- Loading: 0.9 mmol/g; compatible with all solvents (aqueous and organic)
|
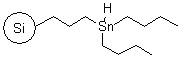
R72530B |
SiliaBond® Tin Hydride |
|
- Versatile, clean and selective solid-bound reagent31,32; the silica support allows toxic and difficult-to-remove organotin by-products to be separated via filtration
- It is used in a variety of transformations including reduction or dehalogenation of alkyl halides33 (SiliCycle 2008 catalogue, page 72), ring enlargement of cyclohexa-dienones, Barton-McCombie deoxygenation of alcohols and Giese reactions
- Loading: 0.6 mmol/g; compatible only with anhydrous and degassed solvents
|
R60530B |
SiliaBond® Tosic Acid |
|
- Silica-bound sulfonic acid that is used as a general acid34 catalyst and solid-supported base scavenger
- Can be used to provide an acidic work-up in the absence of aqueous conditions
- Loading: 0.8 mmol/g; compatible with all solvents (aqueous and organic)
|
R44030B |
SiliaBond Tosyl Chloride |
|
- Reacts with alcohols to yield the bound tosylate, which is used in the synthesis of amines and oxazolines (SiliCycle 2008 catalogue, page 80)
- Loading: 1.0 mmol/g; compatible with anhydrous aprotic solvents, but is not stable in
dimethylformamide (DMF)
|
References
1) Org. Process Res. Dev., 1998, 2, 221
2) J. Catal., 2000, 195, 237
3) J. Catal., 2000, 195, 412
4) Chem. Rev., 2003, 103, 4307
5) Tetrahedron, 2003, 59, 1781
6) Tetrahedron Lett., 1988, 29, 2261
7) Molecular Diversity, 1998, 3, 161
8) Org. Lett., 2002, 4(7), 1167
9) Org. Lett., 2003, 5(24), 4721
10) Synlett, 1998, 625
11) J. Chem. Soc. Perkin Trans. 1, 1989, 105
12) J. Org. Chem., 1991, 56, 1981
13) J. Org. Chem., 1983, 48, 666
14) J. Org. Chem., 1999, 64, 4324
15) Synlett, 2001, 10, 1555
16) J. Polym. Sci., Part A Polym. Chem., 1994, 32, 2457
17) J. Org. Chem., 1989, 54, 5387
18) Tetrahedron Lett., 2001, 42, 2141
19) Synlett, 1999, 10, 1630
20) Synth. Commun., 1996, 26, 225
21) J. Org. Chem., 1993, 58, 2509
22) J. Chem. Edu., 1999, 76, 974
23) J. Org. Chem., 1983, 48, 666
24) Liebigs Ann. Chem., 1993, 173
25) J. Org. Chem., 1992, 57, 3867
26) J. Chem. Soc. Perkin Trans. 1, 1982, 1967
27) J. Chem. Soc. Chem. Commun.,1993, 7, 651
28) Tetrahedron, 1979, 35, 1789
29) Angew. Chem. Int. Ed., 1997, 36, 2661
30) Micro. Meso. Mater., 2000, 35-36, 143
31) Green Chem., 2001, 3, 71
32) J. Am. Chem. Soc., 1997, 119, 6949
33) J. Org. Chem., 1991, 56, 5971
34) J. Org. Chem., 1989, 54, 5437
 
|
|
|


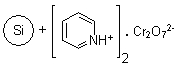
![]()
![]()